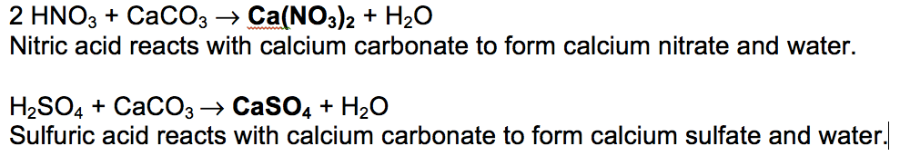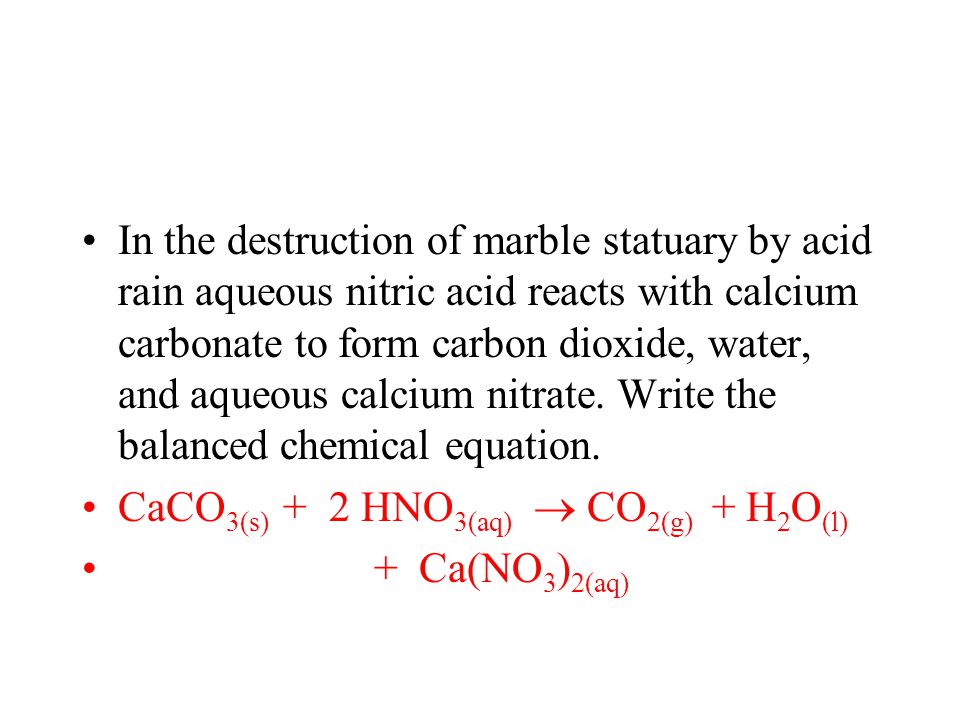Nitric Acid And Marble Equation
Acid rain is caused by nitrogen oxides and sulfur dioxide produced by both natural processes and the combustion of fossil fuels.
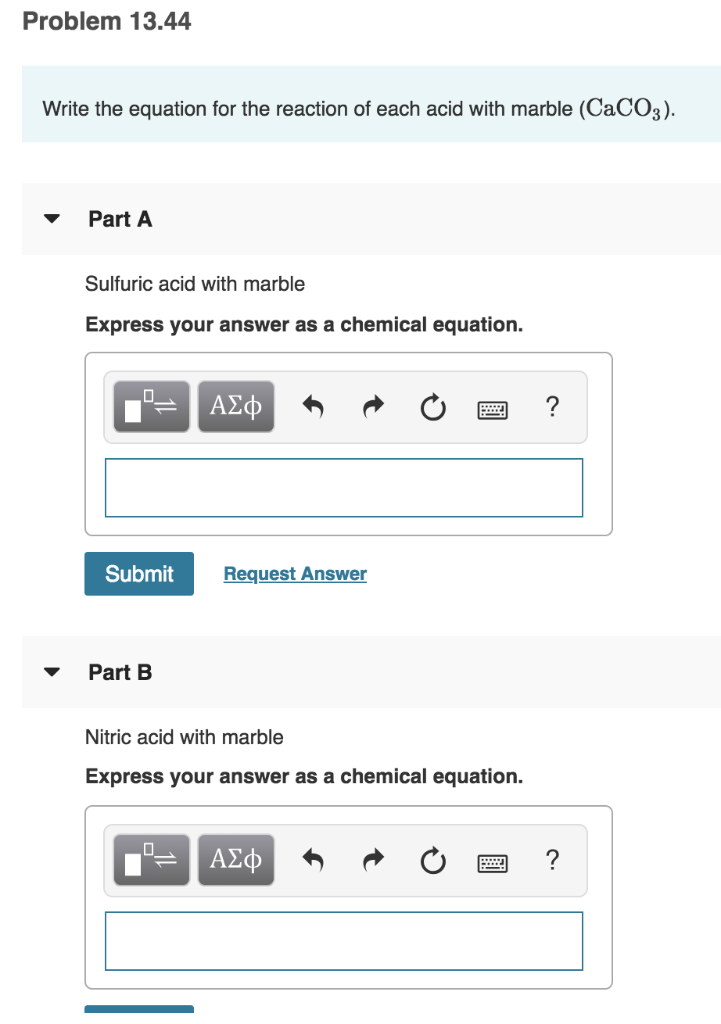
Nitric acid and marble equation. Acid rain contains carbonic acid nitric acid and sulphuric acid co2 no2 and so2. The balanced equation is. When sulfurous sulfuric and nitric acids in polluted air and rain react with the calcite in marble and limestone the calcite dissolves. The rate of reaction between nitric acid and marble chips.
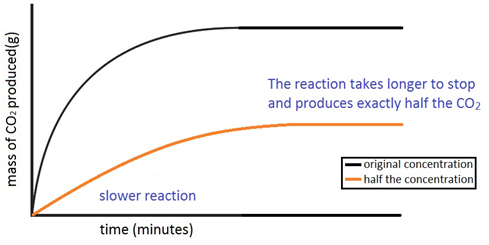
Acid rain is caused by nitrogen oxides and sulfur dioxide produced by both natural processes and the combustion of fossil fuels. This acid dissociates in water to yield hydrogen ions and nitrate ions no 3 in a reaction analagous to the dissociation of carbonic acid shown in equation 2 again lowering the ph of the solution. This means a lower temperature can be used. Stone surface material may be lost all over or only in spots that are more reactive.
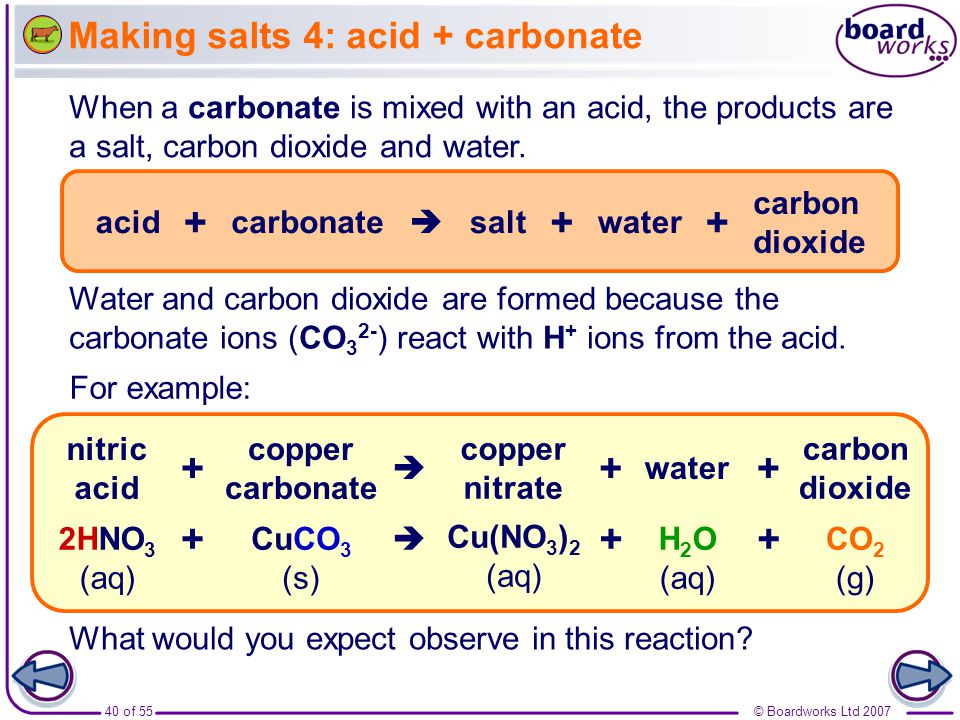
Nitric acid magnesium oxide magnesium nitrate water 2hno 3 mgo mg no 3 2 h 2 o also note that the reaction of metal oxides with acids is exothermic ie heat energy is given out. Catalysts lower the activation energy of reactions making it easier for them to happen. Caco3 2hno3 ca no3 2 co2 h2o this particular reaction is rather slow and so needs to be quicker by changing the factors that affect the rate of reaction. Eventually these oxides react with oxygen and water to give nitric acid and sulfuric acid.
Marble is mostly calcium carbonate caco3 so when it react with nitric acid hno3 the products will be calcium nitrate carbon dioxide which effervesces and water. Caco3 2hno3 ca no3 2 h2o co2 g i believe this reaction happens regardless of the concentration of nitric acid since. In exposed areas of buildings and statues we see roughened surfaces removal of material and loss of carved details. Eventually these oxides react with oxygen and water to give nitric acid and sulfuric acid.
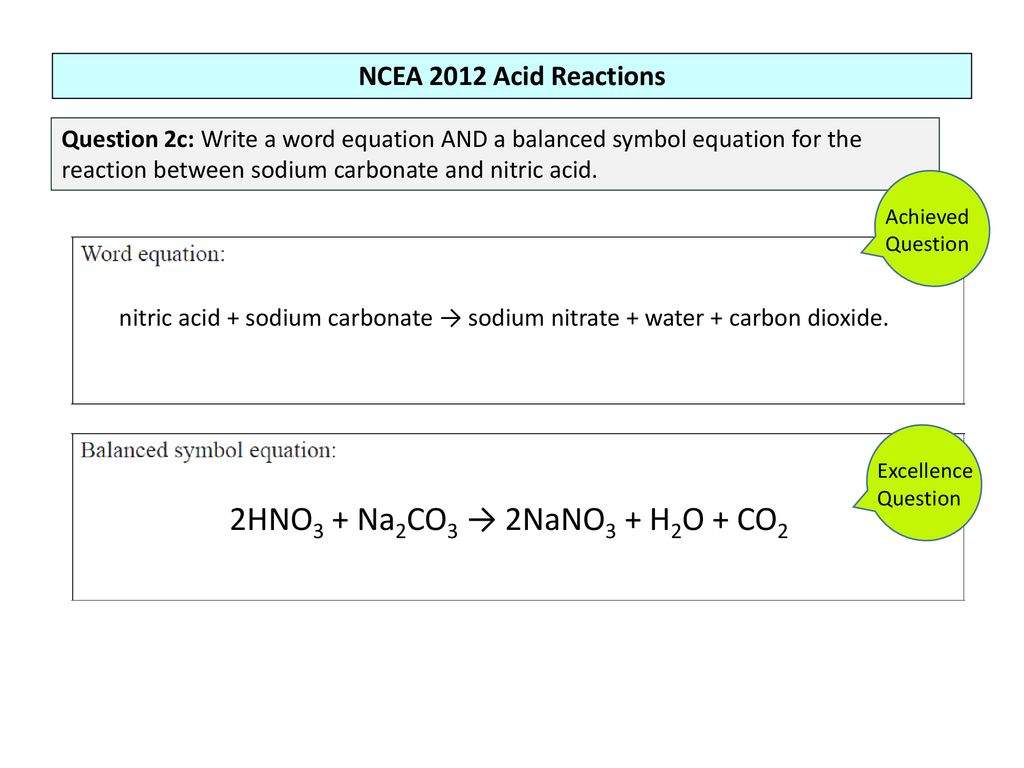
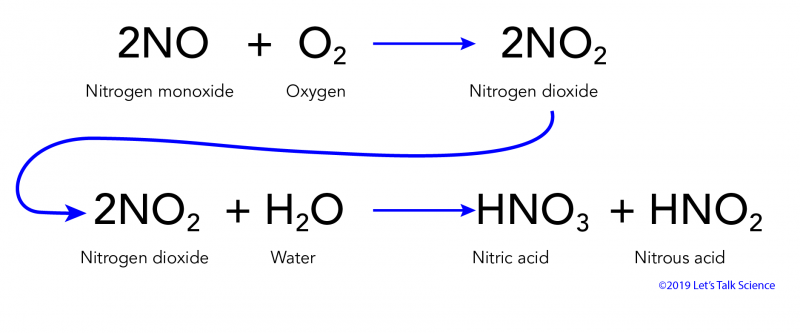
In air no is oxidized to nitrogen dioxide no 2 equation 4 which in turn reacts with water to give nitric acid hno 3 equation 5. Marble is especially sensitive to the degrading by acidic chemicals also to weathering. In addition this helps reduce costs in industrial reactions. Acid rain is one of the top degradation agents for marble artefacts around the world.
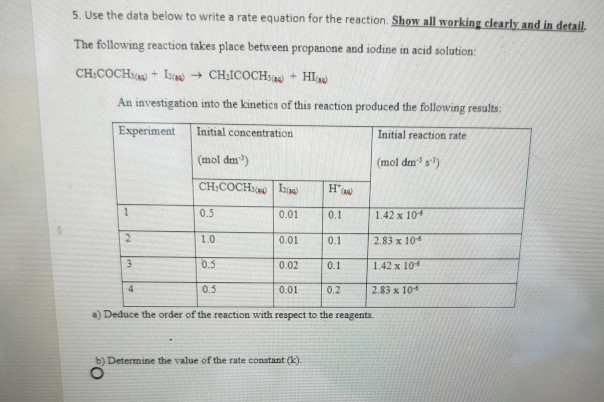
This obviously increases the number of collisions. Acid rain is rainfall whose ph is less than 5 6 the value typically observed due to the presence of dissolved carbon dioxide. The chemical equation that is going to be followed throughout the experiment will be.